Curators Assemble: Behind the Scenes of ‘Superheroes, Science, and the Environment’
Unmasking how a museum exhibition is made.

Unmasking how a museum exhibition is made.
Have you ever wondered about the decision-making processes behind an exhibition? How do curators choose the items you see when you walk through a museum? How do we tell stories using objects?
In honor of Earth Day, which takes place each year on April 22, we’re taking a behind-the-scenes look at our current ExhibitLab, Superheroes, Science, and the Environment. Our ExhibitLab displays are small, focused exhibits that draw on our existing collections to tell new stories. We usually change them several times a year, so there is always something new to see.

For this ExhibitLab, we chose to tell the story of the environment and superheroes. Superheroes permeate American culture through comic books, television shows, and movies. In the last decade, you’ve likely watched a superhero movie, bought superhero toys for your children, or dressed as a superhero for Halloween.
Since its inception, the genre has engaged with contemporary social and political events, including environmental themes. This exhibit presented an opportunity to explore how the world of superheroes addresses challenges such as pollution and waste, while highlighting the strengths of the Institute’s existing collections.

This is the first display case visitors see. It plays an important role in setting the stage for the ideas and themes that emerge as visitors move through the exhibit.
The key item in this case is the Captain Planet field kit. This chemistry set anchors the presentation of superheroes in the exhibit and introduces the idea that even nonscientists can participate in environmental testing and science. The Captain Planet set is part of the Institute’s large collection of chemistry sets; in fact, some of the kit’s predecessors are on permanent display on the second floor of our museum. Aimed at young people, the field kit shows children how to take measurements of their local environments and test water and air quality.

To demonstrate how professional scientists test air and water, we used the Beckman fieldlab oxygen temperature analyzer and photographs of scientists at work in the field and in the lab. These instruments help us link the world of play and learning to scientific research. While the Science History Institute has an extensive collection of environmental instrumentation, this particular device was chosen because, like the Captain Planet kit, it is meant to be used in the field, not in the lab.
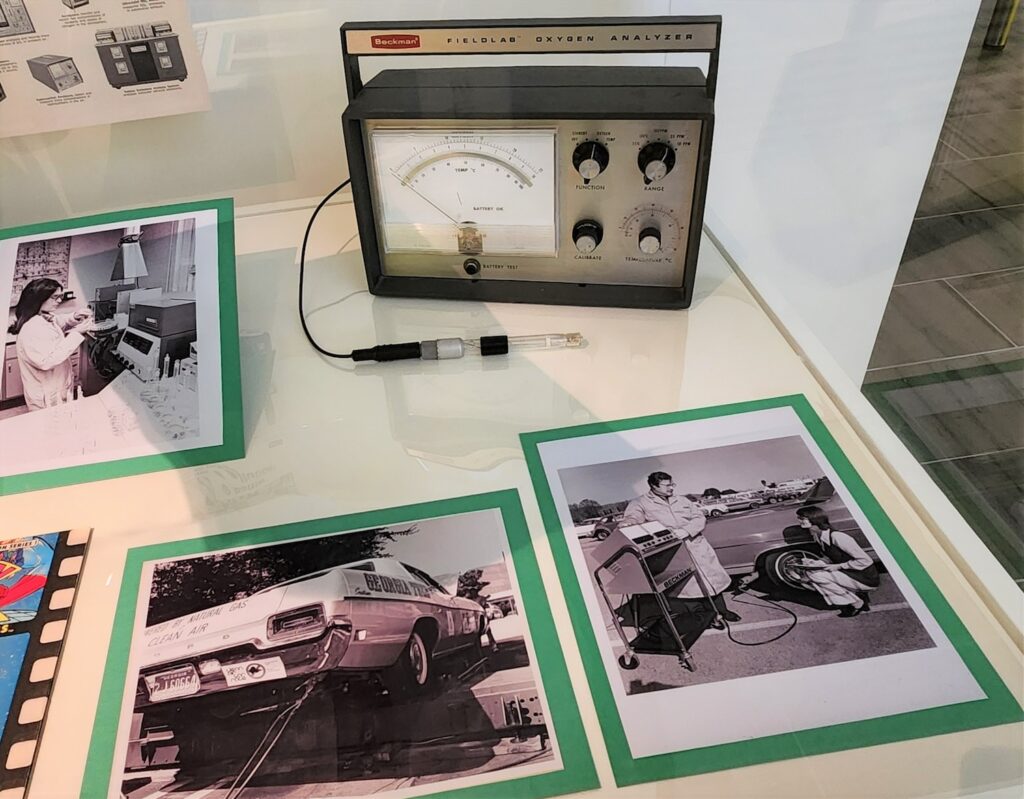
Finally, the Captain Planet comic book introduces a theme that extends through the exhibit. Each of the cases has a comic book that connects the objects and photographs on display to the theme of superheroes. Comic books are ideal for the ExhibitLab format because they are a low-tech way to showcase superheroes we are familiar with from television or movies.
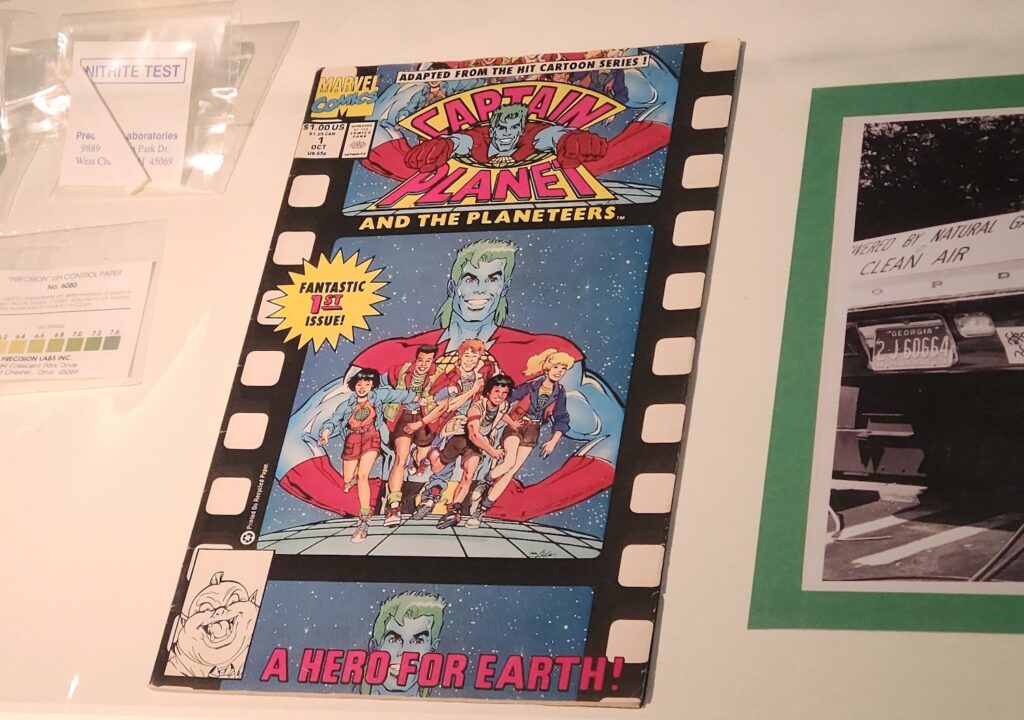
Displaying print comic books rather than showing moving image clips allows us to communicate our theme without relying on objects that might break or malfunction during an exhibit’s run. And of course, comic books are visually striking and appealing to many visitors, especially our younger audiences.

We designed this case around an original copy of Sub-Mariner issue #25 that was published by Marvel Comics in May 1970. On its cover, Namor the Sub-Mariner stands in an undersea world, surrounded by containers of radioactive waste, DDT, noxious gas, insecticide, and chemical spray. Readers of the comic would have understood the significance of the objects immediately—the issue hit shelves just a month after the first Earth Day, an event that drew national attention to the state of the planet.

To build around the cover, we turned to the Institute’s extensive pesticide collection. Selecting objects for display can be surprisingly difficult when there are so many to choose from. How do we choose among them? The one that jumped out the most from the collection was this can of new insect bomb, produced by Chase’s Products.
The clouds and callout graphics evoke the feel of a comic and matched well with the superhero theme of this ExhibitLab and with the comic book cover. The other two bottles round out the case with a splash of color to match the colorful Sub-Mariner cover.
While most people associate DDT (dichlorodiphenyltrichloroethane) with agriculture, manufacturers widely marketed DDT to households for both indoor and outdoor use. These bottles illustrate the ubiquity of the pesticide in the post-World War II United States.

To complete this display, we included a first edition of Rachel Carson’s Silent Spring, the work that brought the dangers of pesticide use to millions of readers. First published in 1962, Carson’s work helped spark the modern environmental movement. Legendary Marvel illustrator Marie Severin might have been thinking of Silent Spring when she included containers of DDT on the cover. We certainly think Rachel Carson qualifies as a real-life superhero!

This case poses a challenge due to its small size: the footprint is barely larger than a sheet of printer paper. To solve this spatial challenge, we decided to tell a story about a big environmental problem with very small parts.
Plastic waste poses a major problem in the environment. Since plastic doesn’t decompose, it breaks down into ever-smaller pieces. Microplastics are pieces of plastic under five millimeters in size, or the size of a sesame seed. The smallest of the broken-down plastics are called nanoplastics, which are so small that they can be breathed in or even carried in the bloodstream. Scientists are still studying the potential impact of nanoplastics on humans and are concerned about the risk of toxicity, endocrine disruption, and cellular inflammation.


To help us tell the tale of microplastics and plastic waste, we recruited a dragon—a Lego dragon that represents plastic waste in the ocean. In 1997 a rogue wave washed over the Tokio Express container ship, sending millions of Legos overboard. Nearly 40,000 of the Legos were dragons, including this one, which we are displaying to the public for the first time.
Of course, Legos do not account for most of the plastic waste in the world’s oceans. To represent the many types of other plastic products, we used a Coke bottle, an advertisement for shopping bags, and a sample of vintage Saran Wrap—all examples of the different types of plastic waste in the world.
To tie in our superhero theme, we included the cover of Great Pacific, a comic book set on the Great Pacific Garbage Patch.

The final case in Science, Superheroes, and the Environment is titled “Superheroes Walk Among Us.” We designed this case after learning about a program at Philadelphia’s Imhotep Institute Charter High School. Imhotep students are helping to provide clean drinking water to communities around the world by distributing 3D-printed water filters.
The STEM ambassadors at Imhotep donated the water filter featured in this display, along with a lab coat, monofilament spool, and photographs. The plastic water bottle represents the latest iteration of their water filters; they are now giving discarded plastic bottles new life as water filters.

The names of the STEM ambassadors and their faculty mentors are listed on the base of the case. Highlighting their work allows us to feature the superheroes in our own community. The final case also “calls back” to Case 1’s Captain Planet environmental field test kit by showing how young people are addressing environmental challenges.
Designing and creating public exhibitions is a balance of history, storytelling, object selection, and artistic choice. We hope you think about these issues the next time you stop by! But hurry: Superheroes, Science, and the Environment is only on view through Saturday, April 27, 2024.
What do Qing dynasty paintings reveal about the secret of degumming ramie?
The Manhattan Project forged a city in the desert at Los Alamos.
Celebrate the Year of the Dragon with some of the legendary creatures in our collections.
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.