Gilbert Newton Lewis
American chemist G. N. Lewis was instrumental in developing the theory of covalent bonding.

The subject of chemical bonding is at the heart of chemistry. In 1916 Gilbert Newton Lewis (1875–1946) published his seminal paper suggesting that a chemical bond is a pair of electrons shared by two atoms.
Once physicists studying the structure of the atom began to realize that the electrons surrounding the nucleus had a special arrangement, chemists began to investigate how these theories corresponded to the known chemistry of the elements and their bonding abilities. Lewis was instrumental in developing a bonding theory based on the number of electrons in the outermost “valence” shell of the atom.
Shared Electrons and Chemical Bonds
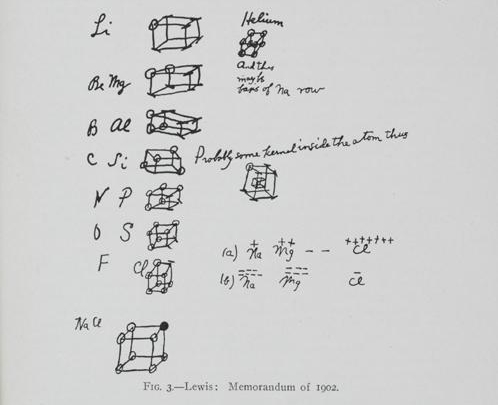
In 1902, while Lewis was trying to explain valence to his students, he depicted atoms as constructed of a concentric series of cubes with electrons at each corner. This “cubic atom” explained the eight groups in the periodic table and represented his idea that chemical bonds are formed by electron transference to give each atom a complete set of eight outer electrons (an “octet”).
Lewis’s theory of chemical bonding continued to evolve and, in 1916, he published his seminal article suggesting that a chemical bond is a pair of electrons shared by two atoms. (General Electric researcher Irving Langmuir subsequently elaborated on this idea and introduced the term covalent bond.) For cases where no sharing was involved, Lewis in 1923 redefined an acid as any atom or molecule with an incomplete octet that was thus capable of accepting electrons from another atom; bases were, of course, electron donors.
Contributions to Thermodynamics
Lewis was also important in developing the field of thermodynamics and applying its laws to real chemical systems. At the end of the 19th century when he started working, the law of conservation of energy and other thermodynamic relations were known only as isolated equations.
Lewis built on the work of another American pioneer in thermodynamics, Josiah Willard Gibbs of Yale University, whose contributions were only slowly recognized. Their work was of immense value in predicting whether reactions will go almost to completion, reach an equilibrium, or proceed almost not at all, and whether a mixture of chemicals can be separated by distillation.
Education and Career
Lewis was educated at home, while his family lived in Massachusetts and Nebraska, until he was 14 years old. His subsequent education was more conventional, although nonetheless stimulating, and included a doctorate from Harvard University earned under Theodore W. Richards.
Lewis then made the pilgrimage to Germany to work with the physical chemists Walther Nernst and Wilhelm Ostwald. He held several university faculty appointments, including ones at the Massachusetts Institute of Technology and the University of California, Berkeley, where he expanded the programs in chemistry and chemical engineering. Lewis was nominated 35 times for a Nobel Prize but never won.
Featured image: Courtesy of The Chemists’ Club.



